By Peter Buczynsky
Functional online inspection systems are among the most critical components of a pharmaceutical packaging line, tirelessly inspecting each component on the line to ensure product compliance and to minimize product liability risk. Therefore, vision system obsolescence is something that should be proactively planned for.
Despite their best efforts to support their product lines, vision system providers are always dependent on availability of components used in their systems. So what happens when these components are no longer available, jeopardizing their support and putting a user’s packaging line at risk of lengthy down time?
Initially, users need to be made aware of equipment obsolescence status, and ideally the supplier can provide this. Typically, components are categorized into several different categories of obsolescence:

Additionally, users should ask their supplier to provide a risk assessment to help understand the history/probabilities of sub-component failures. Following is an example of a risk assessment provided to Pharmaworks customers with Scanware vision systems, showing the probability of failure and the potential extent of impact:
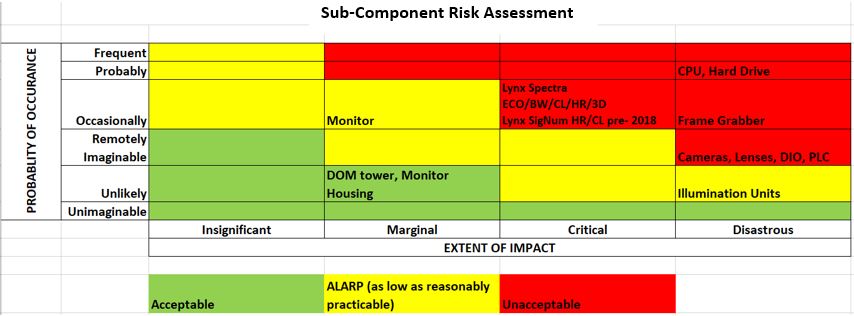
This allows users to make an educated decision based on historical failure information that the OEM has experienced with all of the equipment they have installed.
Vision system obsolescence planning — Incremental upgrades vs. all at once
Consider a user who has multiple packaging lines with various inspection systems from the same OEM. Even though the model names of those systems remained consistent from line to line, chances are high that the sub-components are not identical and, therein, many may already be discontinued. However, there may be compatibility with components across those lines that are still Active/Fully Supported or classified as End of Life. A good example where this applies is with CPU boards. Below, you see an example list Pharmaworks provides to a customer with multiple systems across their packaging lines:
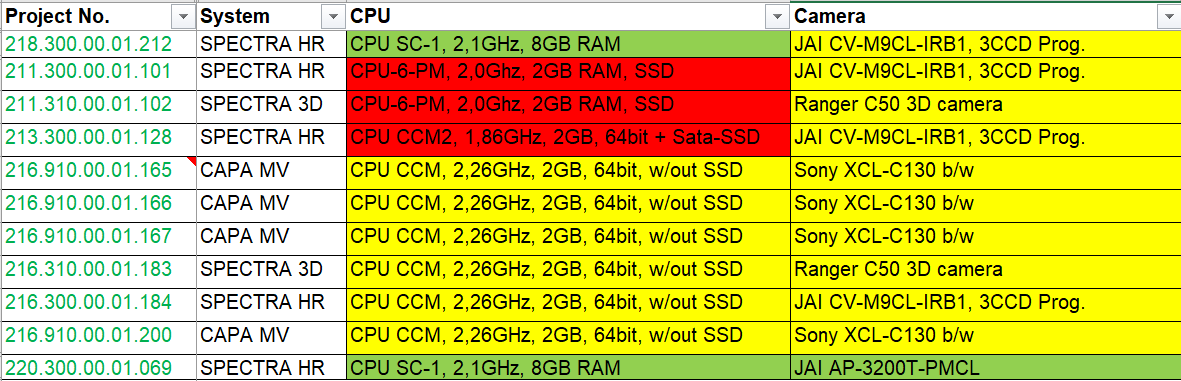
It can be observed that across the lines, there are components (CPU boards and cameras) in various stages of obsolescence. Pharmaworks then advises the customer that rather than replacing all of the CPU boards, for example, they could proactively upgrade one of their lines with an Active/Fully Supported CPU board, which gives them a spare board in their inventory in case one of the other systems were to go down. This allows the customer to budget for future upgrades that fit their production schedule. From the risk assessment, the customer can see that cameras are at a lower risk than CPU boards, so they may choose to obtain a spare camera still in the End of Life stage to place into their inventory.
Additional important information for the user to glean is whether or not previous recipes/formats from inspection systems remain compatible. Typically camera replacements nullify those formats, however CPU boards may allow existing formats to still be compatible. Users should make sure to get this is writing from the OEM to help the validation process, since these are not directly “like for like” replacements.
Here is an example of a tailored obsolescence report configured for a customer that identifies that customer’s existing systems, status of the product lines and sub components, risk analysis, upgrade options, budgeted costs, recommendations to consider, impact of component replacement, typical Q&A and next steps. This allows for a concise summary for the customer with all of the information necessary for preventative risk reduction (and hopefully a better night’s sleep).


–
Pharmaworks has been integrating vision systems onto existing production lines and ones built in-house for over 25 years. As the North American representative for Scanware vision systems, Pharmaworks is unique, being a machine builder, and vision system supplier that takes responsibility for the complete systems integration and life-long systems support. Pharmaworks has in-depth experts in-house that can ensure customers’ production lines keep running. They can replace any existing vision system, taking responsibility for the full electrical and mechanical integration and support users for decades to come.
For more information on Pharmaworks’ online vision inspection technology, please contact the author of this article, Peter Buczynsky.
